
Thermal Energy, Heat, and Temperature
Thermal Energy: Matters are made up of atoms. And, each atom in the matter is vibrating with some energy i.e., each atom has some kinetic energy. The total sum of the kinetic energy of the molecules contained in a body is called Thermal Energy.
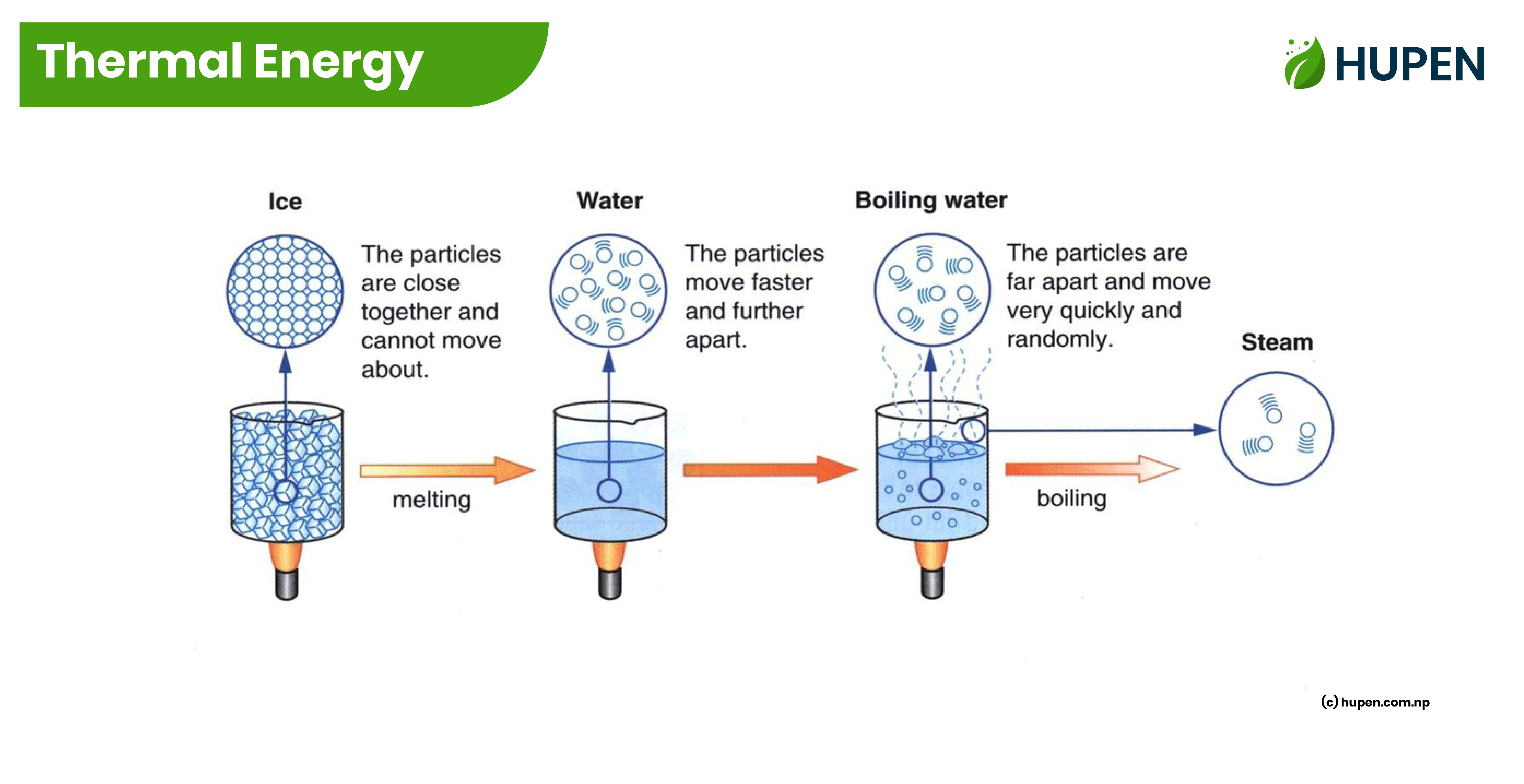
Temperature: The measurement of the hotness or coldness of an object is called temperature. The intensity of molecular vibration is called temperature. Its SI unit is Kelvin (K). The temperature of a body is measured with the thermometer. Hence, the intensity of molecular vibration is directly proportional to the temperature.
So, Kinetic Energy a Temperature
Heat: Heat is the quantity of thermal energy that transfers from one part to another due to the temperature difference. Its SI unit is Joule (J). The heat contained in a body is measured with a calorimeter.
Absolute Zero Temperature: Absolute zero temperature is the lowest possible theoretical temperature at which all atoms are supposed to have zero thermal energy. In other words, the atoms are supposed to be at rest at this temperature. It is -273.150C or 0 K.
Effects of Heat on Volume
When an object is heated, the molecular vibrations within it intensify. Consequently, most substances expand when subjected to heat and contract when cooled.
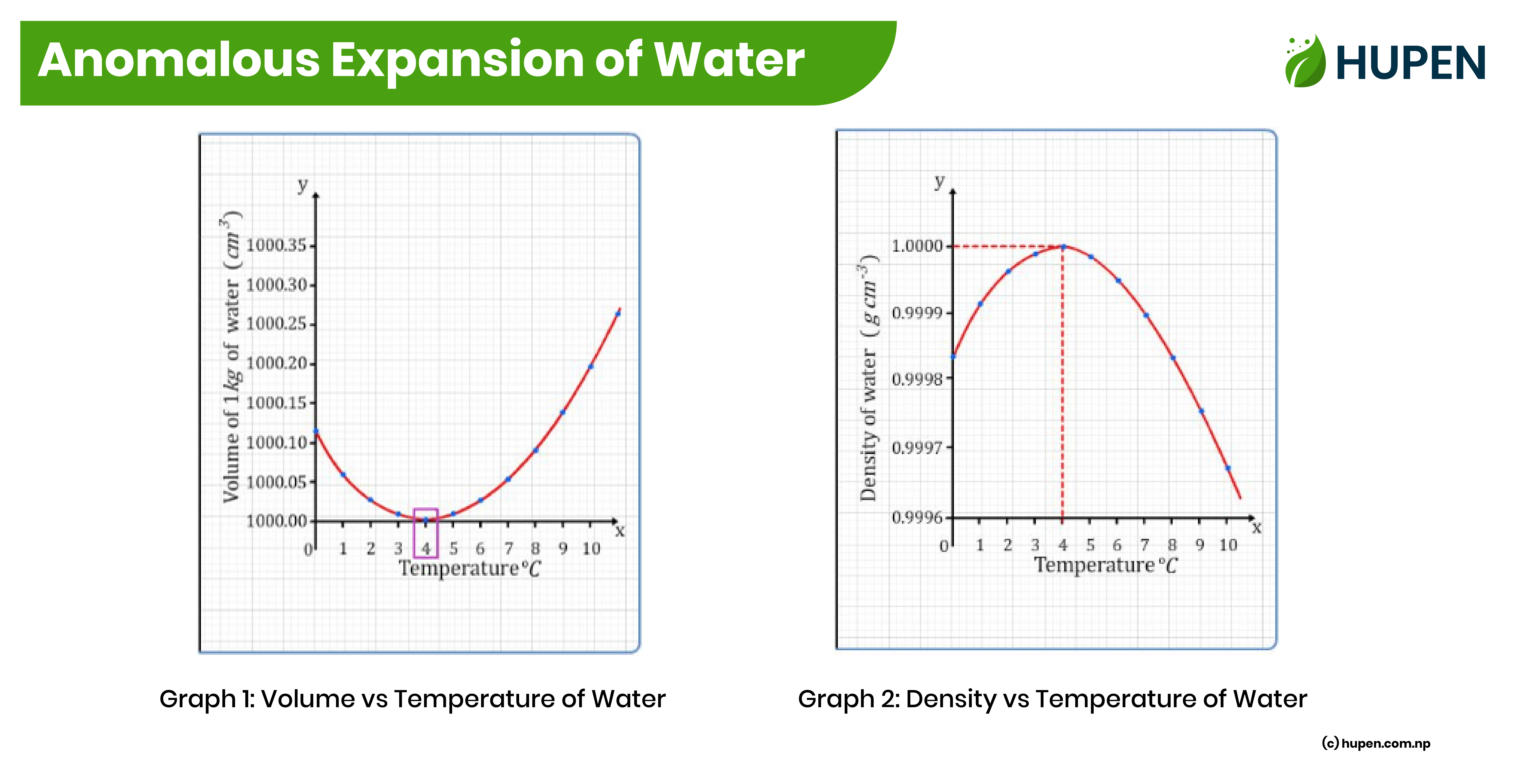
Anomalous Expansion of Water
Water behaves differently compared to other substances when it gets colder or hotter. Most things shrink when they get colder and expand when they get hotter, but water does something strange. When it gets colder, it shrinks until it reaches around 4 degrees Celsius (39.2 degrees Fahrenheit). After that, if it gets even colder, it starts to expand instead of shrinking. This makes the water less dense. So, the densest point for water is at 4 degrees Celsius.
Heat Equation
The quantity of heat lost or gained by the object is equal to the product of its mass, specific heat capacity, and change in temperature. It is called the heat equation i.e.
Verification of heat equation
Let us consider m be the mass of an object, dt be the change in temperature and Q be the quantity of heat lost or gained by the object. Then
- The quantity of heat lost or gained (Q) is directly proportional to the mass (m) of the object.
- The quantity of heat lost or gained (Q) is directly proportional to the change in temperature (dt).
Specific Heat Capacity
The amount of heat required to increase or decrease the temperature of an object of 1 kg mass by 10C is called specific capacity. Its SI unit is J/kg0C
Advantages of High Specific Heat Capacity of Water:
- A hot water bag is used during injury.
- Water is used as a coolant in machines and vehicles.
- During high fever, a cloth soaked with water is used on the forehead.
The specific heat capacity of some substances.
| SN | Substances | Specific Heat Capacity | SN | Substances | Specific Heat Capacity |
| 1 | Water | 4200 | 6 | Aluminum | 887 |
| 2 | Ethyl Alcohol | 2400 | 7 | Iron | 460 |
| 3 | Kerosine | 2100 | 8 | Copper | 385 |
| 4 | Ice | 2100 | 9 | Silver | 236 |
| 5 | Mercury | 126 | 10 | Gold | 130 |
Measurement of Temperature
The device used to measure the temperature of an object is called a thermometer. The working principle of the thermometer is “The increase in volume is directly proportional to the rise in temperature”. There are different types of thermometers.
- Liquid in Glass Thermometer:
- Digital Thermometer:
- Radiation Thermometer:
Calibration Of Thermometer
The process of determining of upper fixed point and lower fixed point on a newly constructed thermometer to make a scale is called calibration of the thermometer. In the very beginning, two fixed points upper fixed point and a lower fixed point are determined.
Upper Fixed Point: The temperature at which the pure water boils at standard atmospheric pressure (sea level) is called the upper fixed point. Its values are 1000C, 2120F, or 373K.
Lower Fixed Point: The temperature at which the pure water freezes at standard atmospheric pressure (sea level) is called a lower fixed point. Its values are 00C, 320F, or 273K.
Principle of Calorimetry
Statement
“The heat loss due to radiation being neglected, the quantity of heat lost by one object is equal to the quantity of heat gained by the other object”.
Important formulae related to this chapter
1. Heat Equation
2. Principle of Calorimetry
Share Now
Share to help more learners!

